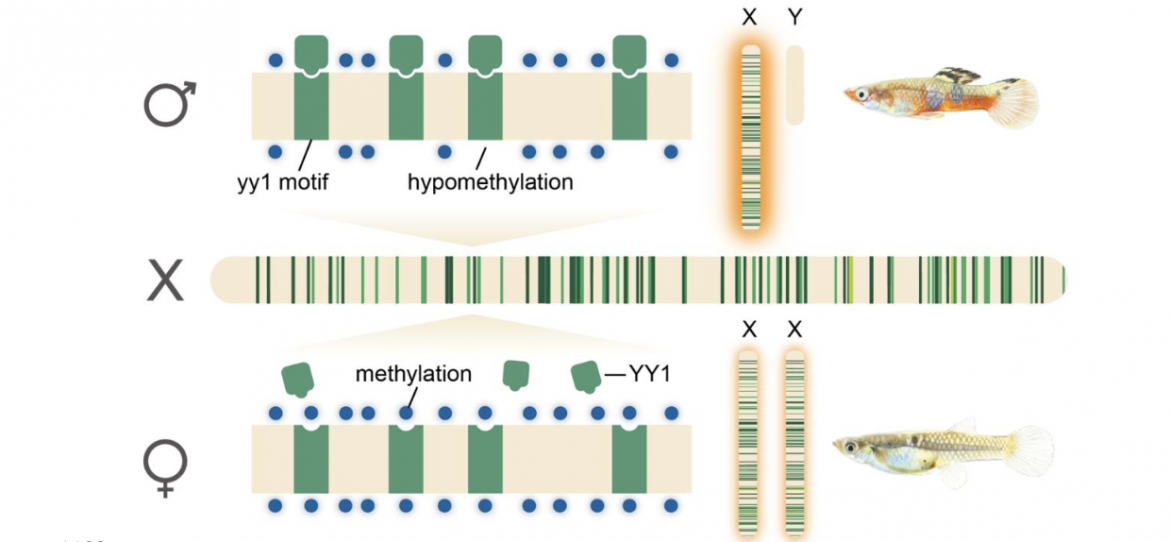
Figure 4. Proposed model for complete X chromosome compensation in P. picta. Hypomethylation of YY1 DNA binding motifs in males promotes male-specific binding of the YY transcription factor and the global upregulation of genes on the X chromosome in males. Hypermethylation of YY1 DNA motifs in females prevents YY1 binding and the hyperexpression of X chromosome genes in females. Green bars indicate position of YY1 motifs along the X chromosome and correspond to the clade of repetitive elements that the YY1 motif is located in based on colors in Figure 3. Illustration by Jacelyn Shu
Abstract
Sex chromosome dosage compensation is a model to understand the coordinated regulation of transcription, however the advanced age of the sex chromosomes in model systems make it difficult to study how the complex regulatory mechanisms underlying chromosome-wide dosage compensation can evolve. The sex chromosomes of Poecilia picta have undergone recent and rapid divergence, resulting in widespread gene loss on the male Y, coupled with complete X chromosome dosage compensation, the first case reported in a fish. The de novo origin of a novel dosage compensation system presents a unique opportunity to discover new mechanisms of gene regulation and their evolutionary origins. By combining a new chromosome-level assembly of P. picta with whole-genome bisulfite sequencing and RNA-Seq data, we determine that the binding motif of Yin Yang 1 (YY1) is associated with male hypomethylated regions on the X, but not the autosomes. The male-specific hypomethylation of these motifs offers a putative model for male specific upregulation of genes on the X. These YY1 motifs are the result of a recent and rapid repetitive element expansion on the P. picta X chromosome, which is absent in closely related species that lack dosage compensation. Taken together, our results present compelling support that a disruptive wave of repetitive element insertions carrying YY1 motifs resulted in the remodeling of the X chromosome epigenomic landscape and the de novo origin of a new dosage compensation mechanism.