HELP FOR PRELAB EXERCISE #1:
THE OSMOTIC AND IONIC ENVIRONMENT OF CELLS
1- ALL MOLECULES MOVE (MORE OR LESS).
In nature, as long as temperature is above the absolute zero (0 Kelvin or -273oC) all molecules possess kinetic energy or energy of motion. In other words, they move, wriggle and giggle at random. The three state of matter (solid, liquid and gas) differ with respect to the freedom of movement of their constituent molecules: this is what makes a solid "solid", a liquid "liquid" and a gas "gaseous".
The molecules of a solid are closely packed and the forces of attraction between molecules allow them to vibrate but not to move around.
In a liquid, the molecules are farther apart, the attractions between molecules are weaker and the molecules move about with considerable freedom.
In a gas, the molecules are so far apart that intermolecular forces are negligible and molecular movement is not restricted at all by neighbouring gas molecules.
Molecules in liquids and gas move around in a perpetual random fashion. They are in constant motion, they move about randomly at high speeds, they collide and ricochet off one another changing direction with each collision. The number of molecules going in a given direction is balanced by the number of molecules going in the other direction and thus even though molecules are moving around they are evenly distributed.
2- HOW DOES WATER DISSOLVE SOLIDS AND LIQUIDS?
(Read Eckert p. 42-44)
The liquid we have to deal with in living system is water. All life forms are made of it and cannot survive without it. The many unusual properties of water that make it so important for living systems are described in your textbook (Eckert p.41-44), so go and read about it if you want to know more. Right now I am just going to talk about the property of water as a solvent.
Water dissolves only solids and liquids whose molecules are electrically charged (e.g. ionic compounds such as salts and polar molecules such as glucose or glycerol).
How does water dissolve a crystal of salt?
![]()
How does water dissolve glucose?
It is very similar to what happens with the salt, except that the glucose molecule does not split into ions.
You can understand now why water cannot dissolve compounds that are completely nonpolar: water has no grip on these molecules to break their bond on each other.
3- SOLUTION - SOLVENT - SOLUTE
A solution is a homogeneous mixture of two or more components that may be gases, liquids or solids. (E.g.: our salt water above is a mixture of water, a liquid and salt, a solid; rubbing alcohol contains two liquids, water and alcohol).
The solvent of a solution is the substance that is present in the greatest quantity (in seawater, the solvent is water. Water is the body's chief solvent)
The solutes of a solution are the substances present in smaller quantity (in saltwater, the solute would be ions Na+ and Cl-, in a solution of glucose, it would be the glucose molecules).
We know also that in a solution, solvent molecules and solute molecules move about randomly and with relative freedom. The only restrictions to the freedom of movement of these molecules are the weak electrostatic attractions that exist between opposite charges on different molecules.
4- CONCENTRATIONS:
just a way to quantify the constituents of a solution.
Concentration of the different solutes of a solution can be expressed in:
g/L: weight of solute / liter of solution
![]()
mole/L of solution or M: Molarity - # of mole of solute molecules / liter of solution
![]()
mole/kg of solvent or m: Molality - # of mole of solute molecules / 1000g of solvent
![]()
Concentration of all the particles dissolved in the solution can be expressed in:
Osmoles/L of solution or Osm:
Osmolarity - Total concentration of all osmotically
active solute particles in the solution -
# of mole of solutes particles / liter of solution -
(Saying that a solution has an osmolarity of 1 Osmol/L
is equivalent to saying that it has a total of
1 mole of osmotically active particles,
NO MATTER WHAT ARE THESE PARTICLES.)
![]()
Osmoles/kg of solvent: Osmolality - # of mole of all the solutes particles / kg of solvent
![]()
It is the TOTAL NUMBER of particles in solution that interest us here - we DO NOT CARE what they are (na+, glucose, Ca++, Cl- or urea): we want to count them all regardless of their species)
Concentration of H+ in solutions of acids and OH- in solutions of bases are expressed in:
N: Normality - # of mole of all the H+ (or OH-) that can be
given by an acid (or a base) in a solution / liter of solution.
![]()
Concentration of positive or negative charge in a solution are expressed in:
Eq/L of solution: Electrical Equivalent/L of solution - # of mole of positive (or negative) charges per liter of solution.
Eq/kg of solvent: Electrical Equivalent/kg of solvent - # of mole of positive (or negative) charges per kg of solvent.
![]()
5- DIFFUSION - OSMOSIS - OSMOTIC PRESSURE:
You know what a solution is, how to quantify it. Now, let have l look at three other concepts:
Simple Diffusion
In a solution, solute particles are continually moving about, colliding with one another and moving off in various directions. The random mixing of solute particles in a solution is called simple diffusion. The flow of solute particles moving in any direction equals the flow of particles moving in the opposite direction.
Net Diffusion
Net diffusion is when the flow of particle moving in one direction is bigger than the flow of particles moving in the opposite direction.
At school you learned that particles diffuse down their gradient of concentration (from a region of high solute concentration to region of low solute concentration). This is an incomplete story: solute particles can move also along along electrical gradient, temperature gradient, pressure gradient etc...
Solute particles move from one place to another because of differences in their potential energy. Solute particles move from a region where their potential energy is greater to a region where their potential energy is lower, regardless of the reason for the potential difference. Potential energy differences between two regions can be caused by differences in pressure, temperature, concentration of solute particles, voltage etc...
In the lab exercise, the gradient of potential energy of solute will be caused by their gradient of concentration (everything else: temperature, pressure... is the same in the two regions). As the concentration of solute particles increases, their potential energy increases. Thus, solute particles will move from the region of higher solute particle concentration (where their potential energy is higher) to region of lower solute particle concentration (where their potential energy is lower).
Like solute particles, Water molecules also move from one place to another because of differences in their potential energy. This is usually referred to as the water potential. Water moves from a region where water potential is greater to a region where water potential is lower, regardless of the reason for the water potential difference. As with solute potential, water potential differences between two regions can be caused by differences in pressure, temperature, concentration of solute particles, etc.....
In the lab exercise, the gradient of water potential will also be caused by a gradient of concentration of solute particles (everything else: temperature, pressure is the same in the two regions). In solutions, water potential is affected by the concentration of dissolved particles of solutes. Note: As the concentration of solute particles increases, the water potential decreases. We know that water molecules move from regions of high water potential to regions of lower water potential. This means that water moves from regions of low concentration of solute particles (high water potential) to regions of high concentration of solute particles (low water potential). As concentration of solute particles increases, the concentration of water molecules per unit volume of solution decreases and vice versa. Thus, one could also say that water moves from regions of high water concentration to regions of low water concentration. This is what you learned in high school. However, from now on, relate the movement of water to the gradient of water potential between regions. Explaining the movement of water as a function of only the gradient of solute particle concentration or water concentration can be misleading in the long run because you will assume automatically that there is no gradient of temperature and pressure (and other factors which affect water potential) and you will forget to take them into consideration when needed.
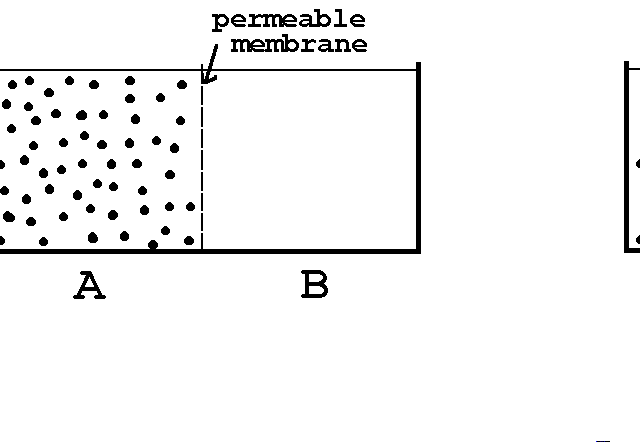
6- OSMOTICITY - TONICITY: