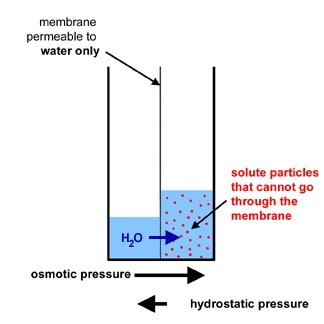
Important Note: As the water rush in, you can see that the volume of water
increases in the compartment
on the right and decreases in the compartment on the left.
- On the right, you still have the same quantity of solute
particles but you have a greater quantity of water.
This means that the concentration of solute
particles (= osmolarity) in the right compartment decreases.
- As the osmolarity in the right compartment decreases, the flow of water
(blue arrow) will decrease (because the difference in gradient
potential for water decreases between the two compartments).
As a consequence, the osmotic pressure will decrease also.
- There is a difference in the height of the columns of
water in the two compartments: the column on the right
is higher than the column on the left.
- This means that the column of water on the right exerts
a pressure (hydrostatic pressure) that opposes the movement of water
from the left side to the right side. In effect, this hydrostatic
pressure contributes to the diminution of the movement
of water from the left side to the right side.
- In this demonstration, hydrostatic pressure opposes osmotic pressure.
