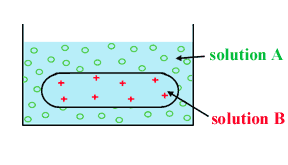
Solutions A is hyperosmotic to solution B.
The solute particles + and
o are non-penetrating.
Will the dialysis bag change shape or not?
1- Identify all the different type of solute particles
in all the compartments of the system.
The solute particles in solution A are o.
The solute particles in solution B are +.
2- Figure out what each type of solute particles "want to do".
The o want to move along
their gradient of potential energy
(in this case along their gradient of concentration).
They want to move in the dialysis bag (into the solution B).
Can they do it? NO -
They are non-penetrating and this means they
cannot cross the membrane of the dialysis bag.
The + also want to move along
their gradient of potential energy
(in this case along their gradient of concentration).
They want to move out of the dialysis bag and into the surrounding solution (A).
Can they do it? NO -
They are non-penetrating and this means they
cannot cross the membrane of the dialysis bag.
Both o and + will stay where they are.
3- Make them do it.
NO WAY!
4- After they have "done it" figure out the
overall concentration of all the solute particles (or osmolarity) in each compartments
Solution A was hyperosmotic to solution B to start with.
This means that the [solute particles] in solution A is higher than in solution B.
As no solute can cross
the dialysis membrane, it will stay this way.
5- predict the movement of water
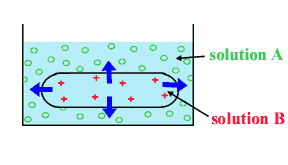
The potential energy for water is higher inside the dialysis bag
than inside because there is less solute particles per unit of volume
that will disturb the 3-Dimentional arrangement of the water molecules.
This means there
is a net movement of water along the gradient of its potential
energy from inside the dialysis bag to outside. This movement of water will
cease only when equilibrium is reached (same potential energy for water on either side of the dialysis membrane).
6- figure out how the shape of
the dialysis bag will be affected.
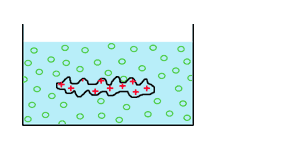
The dialysis bag will shrink ---> The solution A is hypertonic.



