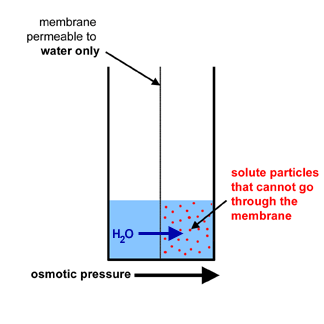
In the compartment on the right we are going to put a
red solute.
The red dots are the solute particles in solution.
Important Note: Everything is the same in both compartments except for the
concentration of solute particles.
This means that the difference in
potential energy for the solute particles and for the water between the right and left compartments
are determined by the difference in their concentration of solute particles (=osmolarity)
.
- The potential energy for the solute particles is high in compartment on
the right and low in the compartment on the left.
- The red solute particles want to move down their gradient of potential energy
(from high to low).
Can they do it? NO because they cannot cross the membrane between the compartments.
- Now consider the water:
- There is less entropy for water in the compartment on the left than on the compartment on the right.
- This means that the potential energy of water is high in the left compartment and
low in the right compartment.
- The water wants to move down its gradient of potential energy (from high to low).
Can it do it? YES because it can cross the membrane between the compartments.
- You will see a net movement of water (osmosis) from the left compartment
to the right compartment (blue arrow).
- A very strong osmotic pressure (black arrow) will be exerted in the compartment on the right
by the water rushing in.
