
 |
Diagram of Acinar cell showing zymogen granules (Black); ER, Golgi, nucleus etc. The cell surface facing the duct is at the top of the diagram. |

In this experiment a pulse of 3H-lableled amino acid is given, followed by a cold chase.
Cells are then fixed at various later times, and sections are cut.
The sections are prepared for autoradiography.
Analysis of pictures of successive stages shows progression of the protein: ER, Golgi, Condencing Vesicles, Zymogen granules
Here is a time course
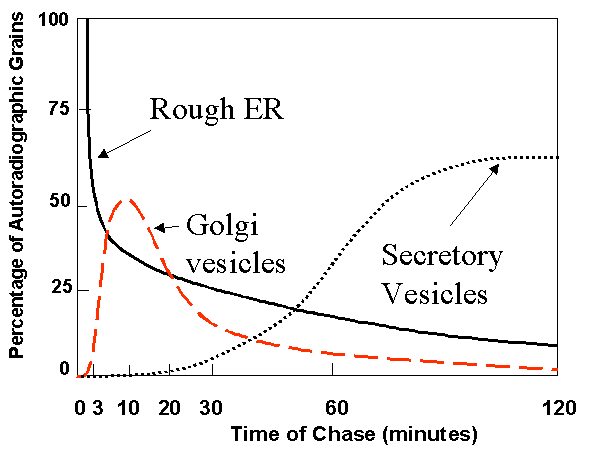
Note that label moves through the secretory pathway from ER to Golgi to condensing vesicles to zymogen granules.
See Pulse - Chase self-test in quiz module.
Make sure that you understand this experiment.
Questions:
1. Would you expect the properties of the cisternal side of the Golgi membranes to me more similar to the extracellular or to the cytosolic side of the plasma membrane? Why?
2. If a slice of pancreatic tissue had been incubated in 3H-leucine continuously for 2 hr, where would you expect to find incorporated radioactivity in acinar cells?
3. Can you design an experiment to test the opposing models for Golgi function (cisternal maturation vs. vesicle transport models). Suppose you could insert an inert fluorescent bead into the cis cisterna of the Golgi complex of a cultured cell.
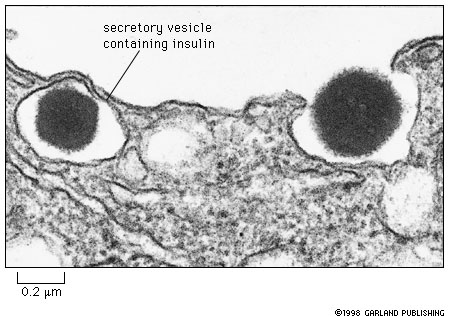
Insulin is a peptide hormone that is secreted by beta cells of the pancreas into the blood. This figure (14-26 in your text) shows two dense core secretory granules. The one at the left is ready to be secreted and the one on the right is in the act of being secreted; the vesicle containing the granule has fused with the plasma membrane.
The diagrams below show a domain map of the insulin protein as it is first synthesized. The disulfide bridges are added in the ER during the early stages of the processing of the protein.

The protein that is secreted consists only of two short polypeptide chains joined by disulfide bridges. The entire central part of the original molecule is missing.
This is an example of processing of a protein by proteolytic cleavage. This occurs commonly in the case of many secreted proteins.
This case study is about how to study proteolytic processing of a protein.
What are the main questions would you need to ask?
How could you get information to answer the key question?
Here is an experiment that was done to answer the question, "When and where does cleavage of the molecule occur?"
|
Antibody to proinsulin
|
Antibody to insulin
|
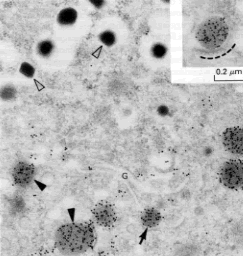 |
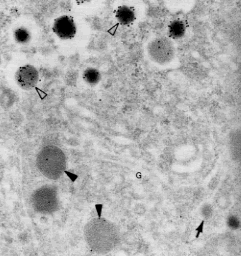 |
Endocytotic Pathway
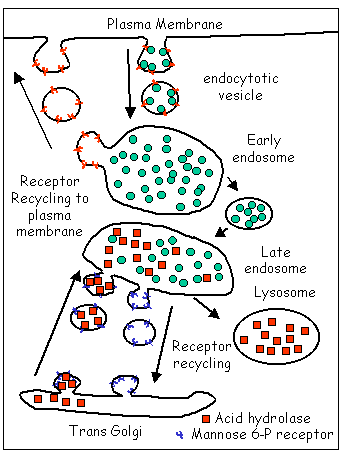 |
Cargo (ligands) taken in by endocytosis is
shown as green circles.
The hydrolytic enzymes of the lysosomes are shown as red squares. Notice that the surface cargo receptors (red Ys) are recycled as are the mannose- 6- phosphate receptors (blue Ys) that are responsible for bringing the lysosomal enzymes to the late endosome. |
Scenarios:
A. Trace a protein molecule that is taken up and digested by a cell, starting when the protein molecule is outside the cell, and continuing till the protein is destroyed in a lysosome. List in order all of the major events and processes.
B. Trace a molecule of the lysosomal enzyme acid phosphatase from its origin on a ribosome in the cytosol until it is finally in place in a secondary lysosome. List in order all of the major events and processes.
C. Trace a molecule of cargo receptor for receptor mediated endocytosis at the plasma membrane from before it binds its ligand until it is returned to the cell surface. List in order all of the major events and processes.
D. Trace a molecule of mannose-6 phosphate receptor from initiation of its synthesis on a ribosome in the cytosol until it has been recycled to the Golgi apparatus. List in order all of the major events and processes.