
1. (True/ False) Like the lumen of the ER, the interior of the nucleus is topologically outside of the cell.
2. What is the fate of a protein with no sorting (targeting) signal?
3. (True/ False) Membrane-bound and free ribosomes, which are structurally and functionally identical, differ only in the proteins that they are making at a particular time. Explain.
4. Imagine that you have engineered a gene, encoding a protein with a pair of conflicting signal sequences,
If the protein was produced by a cell, predict which signal would win out. Explain your reasoning.
5. Co-translational Insertion of membrane Proteins
A. Make a map showing the location of signal sequences, start transfer sequences and stop transfer sequences in protein A and protein B below that end up inserted in the membrane as shown.

Protein A
![]()
Protein B
![]()
B. Make a map for the arrangement of signal sequences, start transfer sequences and stop transfer sequences for a double pass protein with the N terminal end a) in the cytosol, b) in the ER lumen.
6. Do you understand insertion of membrane proteins? -
Match the following arrangements of proteins in the membrane with the appropriate polypeptide domain maps below:

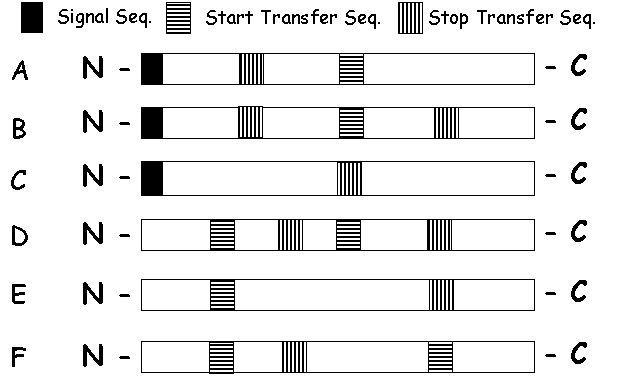
 |
8. This figure is an average hydropathy plot for a protein. N terminal end is amino acid number 1 (left). The hydropathy index is an estimate of the hydrophobicity of a running average of three sequential amino acids within the sequence. Given that amino acid 1 represents the first codon of the open reading frame for this protein, make a sketch of how this protein would be arranged in the membrane. Be sure to label the N terminal and C terminal ends of the protein, and the cytosol and lumen sides of the membrane. |
CF patients lack a Cl- channel in epithelial cells of lung, sweat glands etc.
What could be wrong with the mutants? Make a short list of alternatives:
The most common mutations result in lack of the channel protein in membrane. Normal amounts of protein are produced. The mutant protein enters the ER properly, but never gets out. The problem is that the protein is not folded properly.
8. (True / False) all of the glycoproteins and glycolipids in intracellular membranes have their oligossaccharides facing the lumen side, whereas those in plasma membrane have their oligosaccharides facing the outside of the cell. Explain.
9. Membrane conformation SER vs. RER. Making hypotheses.
10. Interpretation of Golgi Images.
Until recently there were two competing models for how material progresses through the Golgi apparatus.
 |
In the cisternal progression model, new cisternae
form continuously as vesicles from the ER coalesce at the cis face
of the Golgi. Each newly formed cisterna moves through the stack with appropriate
modification of its contents, and finally breaks up into transport vesicles
at the trans face. (Fig A) |
 |
In the vesicle transport model, cisterane remain fixed and the maturing glycoproteins move from the cis to the trans cisternae in transport vesicles. (Fig B).
|
One experimental test of these models involved following a protein (protein A) that is normally glycosylated with both
The experiment made use of mutant cells that are defective in
the addition of galactose to glycoproteins, and normal cells that
could add galactose to glycoproteins.
In each experiment some cells were labelled with radioactive
glucose. These cells were then fused with non-labelled cells.
The fused cells contained Golgi of both types (with and without labelled glucose).
A few minutes after fusion the cells were dissolved in detergent
and the protein being studied (protein A) was isolated.
This protein was separated into two fractions, molecules with attached galactose and molecules without galactose.
The fraction with attached galactose was precipitated (pellet),
leaving the rest of the protein in solution (supernatant).
The following distribution of label was observed in control
and experimental cell combinations:
|
Cell combination |
Fraction of label
in pellet (protein with galactose) |
Fraction of label
in supernatant (protein without galactose) |
|
Labeled wild-type cells fused with non-labeled wild- type
cells |
85% |
15% |
|
Labeled mutant cells fused to non-labeled mutant cells |
5% |
95% |
|
Labeled mutant cells fused with non-labeled
wild-type cells |
|
55% |
With this information, answer the following questions:
1. Movement of proteins between which two compartments of the Golgi apparatus is being tested in the experiment? Explain your answer briefly.
4. Explain which model is supported by the results in the table?
5. Do you see any problems with this experiment?
A second test of the vescular transport and cisternal maturation models used EM to follow movement of protocollagen through the Golgi apparatus. Protocollagen was identified by specific interaction with an antibody.Protocollagen is assembled in the cis cisterna of the Golgi apparatus
If transport of this protein from the ER was specifically blocked, protocollagen disappeared in the following order:
If the block was maintained until the Golgi was free of protocollagen, and the block was then removed so that protocollagen could again be transported to the Golgi, then protocollagen reappeared:
Which model of Golgi dynamics - vesicular transport or cisternal maturation do these results support? Explain why.
13. Most exported proteins move across the Golgi stack in 5-10 minutes. However, large proteins, like protocollagen, take about 1 hr. What do you make of this? What asumptions do you have to make to explain the results?
14. What is the original evidence for cisternal maturation?